Trainee Poster Day 2014
2014, 7th Annual Trainee Poster Day
In its seventh year, our Poster Day retreat has become a spotlight event for our group. Hosted again at the Griggs Boathouse on the Scioto River, we brought our faculty and trainees out of the lab to share and discuss their research and build new connections. Nearly 40 postdocs and graduate students from 18 different labs presented their research projects to the group over a potluck lunch and the beautiful sights. Listed below are this year’s posters.
-
- Taylor Banh (Belury Lab) – “The role of an adiponectin mimetic in the treatment of mice with cancer cachexia”
- Michael Berberoglu (Amacher Lab) – “Pax3/7-positive satellite-like cells, expressing novel satellite cell markers Rbfox1l and Rbfox2, participate in muscle regeneration following injury of adult zebrafish skeletal muscle”
- Alisa Blazek (Weisleder Lab) – “Exercise-mediated upregulation of follistatin-like 3 expression following treadmill walking is insufficient to increase muscle contractile force”
- Jessica Chadwick (Rafael-Fortney Lab) – “Elucidating the Role of Mineralocorticoid Receptors in Skeletal Muscle as a Potential Therapeutic Target for Duchenne Muscular Dystrophy”
- Daniel Comiskey (Chandler Lab) – “Splicing Factor SRSF2 Positively Regulates the Alternative Splicing of MDM2”
- Scott Crawford (Best Lab) – “Mechanical Work Associated with Massage in a Rabbit Model Following Eccentric Exercise Injury”
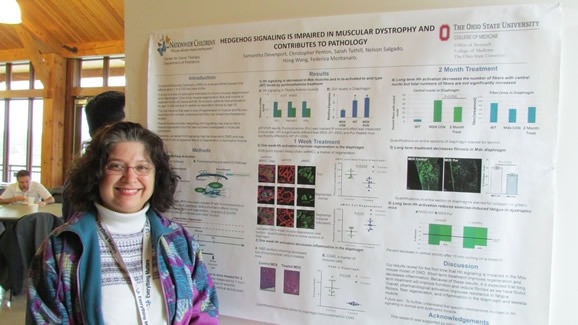
- Sammi Devenport (Montanaro Lab) – “Reduced Hedgehog Signaling in Muscular Dystrophy Impairs Muscle Regeneration and Function”
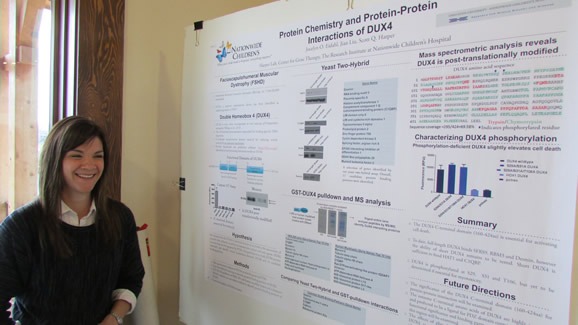
- Jocelyn Eidahl (Harper Lab) – “Protein Chemistry and Protein–Protein Interactions of DUX4 “
- Sara Gombash Lampe (Foust Lab) – “Smn Reduction in the Enteric Nervous System Results in Neuromuscular Transmission Defects and Gastrointestinal Dysmotility”
- Jinmo Gu (Guttridge Lab) – “NF-kB function in neonatal muscle development”
- Liubov Gushchina (Weisleder Lab) – “Therapeutic Effect of Modulating Membrane Repair in a Mouse Model of Limb–Girdle Muscular Dystrophy Type 2B”
- Spencer Hauck (Rafael-Fortney Lab) – “Claudin-5 Expression and Cardiomyopathy, and Mineralocorticoid Receptor in Duchenne Muscular Dystrophy”
- Kim Hromowyk (Amacher Lab) – “Unraveling the control and regulation of vertebrate muscle cell fusion using the zebrafish model”
- Chitra Iyer (Burghes Lab) – “Increasing SMN in neurons is sufficient to rescue a severe mouse model of Spinal Muscular Atrophy”
- Aishwarya Jacob (Chandler Lab) – “Regulation of MDM2 alternative splicing: balance of positive and negative interactions between cis elements and trans factors”
- Stephen Kolb (Kolb Lab) – “NeuroNEXT SMA Infant Biomarker Study: Progress Report”
- Priya Londhe (Guttridge Lab) – “Microvesicles containing miRNAs induce cell death and block differentiation of skeletal muscle cells in cancer cachexia”
- Heather Manring (Weisleder Lab) – “Role of Tripartite motif family of E3 ubiquitin ligases in muscle atrophy”
- Jennifer Peterson (Guttridge Lab) – “NBD delivery improves the disease phenotype of the golden retriever model of Duchenne muscular dystrophy”
- Jennifer Petrosino (Ziouzenkova Lab) – “Maximizing the max test: A method for determining cardiorespiratory fitness and dysfunction in mice.”
- Eric Pozsgai (Rodino-Klapac Lab) – “Beta-sarcoglycan gene transfer leads to functional improvement in a model of LGMD2E”
- Corey Ruhno (Burghes Lab) – “Alternative Splicing in Spinal Muscular Atrophy”
- Hussam Salhi (Biesiadecki Lab) – “Cardiac Troponin I Ser-23/24 and Tyr-26 phosphorylation crosstalk”
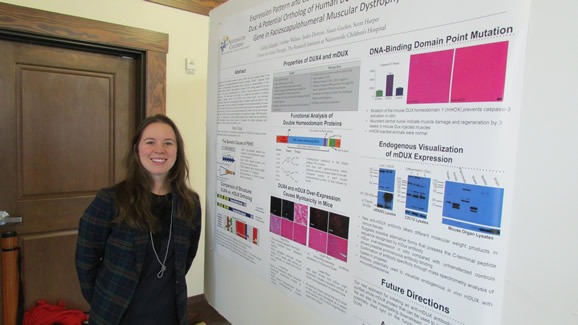
- Carlee Schaefer (Harper Lab) – “Expression Pattern and Gene Characterization of Mouse Dux: A Potential Ortholog to Human DUX4, The Putative Gene in Facioscapulohumeral Muscular Dystrophy”
- Eric Schultz (Janssen Lab) – “Myocardial Contractile Dysfunction Precedes Damage in a Mouse Model of Limb-Girdle Muscular Dystrophy-2F and is Prevented by Exogenous Claudin-5 Expression”
- Vikram Shettigar (Davis Lab) – “Engineering cardiac Troponin C: Potential therapeutic for heart failure”
- Jonathan Shintaku (Guttridge Lab) – “Alternative NF-kB regulation of skeletal muscle metabolism”
- Jalal Siddiqui (Davis Lab) – “Modeling the response of cardiac troponin C to calcium on the thin filament: effects of disease-related and post-translational modifications”
- Tabitha Simmons (Flanigan Lab) – “Dose escalation studies of rAAV9 U7snRNA targeting exon 2 show highly efficient skipping in the Dup2 mouse”
- SungWon Song (Kaspar Lab) – “Sustained expression of MHC class I protects motor neurons from ALS astrocyte toxicity”
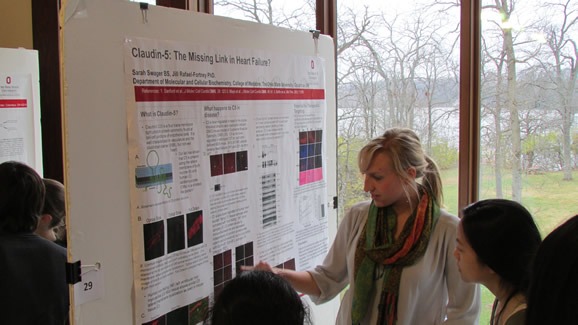
- Sarah Swager (Rafael-Fortney Lab) – “Claudin-5: The Missing Link in Heart Failure?”
- Erin Talbert (Guttridge Lab) – “Satellite Cells Expand in Muscle of Pancreatic Cancer Patients”
- Jared Talbot (Amacher Lab) – “What triggers zebrafish fast muscle morphogenesis?”
- Kiel Tietz (Amacher Lab) – “Rapid clearance of oscillating transcripts during somitogenesis requires the decay adapter Pnrc2 and spliceosome component Cdc5l/Cef1”
- Lindsey Wallace (Harper Lab) – “Dux4 Promoter mice: the next generation”
- Shane Walton (Davis Lab) – “Characterization of the Calcium-binding and Peptide-binding Properties of Arrhythmogenic Calmodulin Mutants”
- Nico Wein (Flanigan Lab) – “Induction of the N-truncated dystrophin by out-of-frame exon 2 skipping prevent or restores muscle function in the Dup2 mouse, providing further support for a therapeutic pathway for 5’ DMD mutations”